mp3®
The injectable Dual-Phase xenograft
The product
mp3® is a Dual-Phase pre-hydrated biomaterial. It is possible to skip the hydration phase and decrease the risk of accidental exposure of the material to pathogens during the manipulation phase.
Dual-Phase granules are endowed with characteristics very similar to autogenous bone¹, and can be used as a viable alternative to AB providing similar performances over time². mp3® granules are gradually resorbable³⁻⁴, and preserve the original shape and volume⁵.
mp3® is made of a collagenic bone mix pre-hydrated with collagen gel.

Source: Politecnico di Torino, Torino, Italy
Histology illustrating the healing after 6 months from mp3® grafting in human. Newly formed bone (#) can be detected around and in between Dual-Phase bone granules (*).
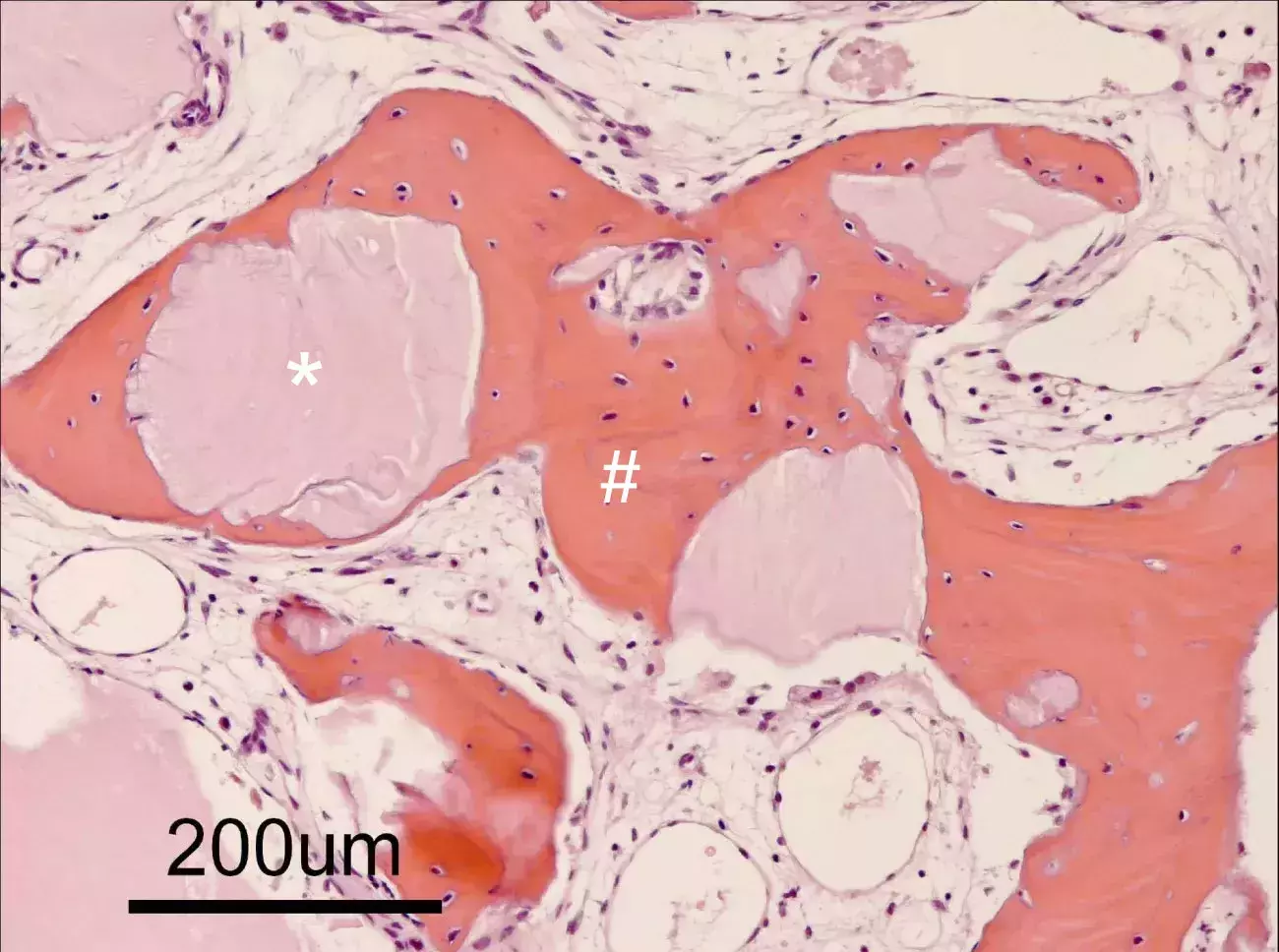
Courtesy of Prof. Ulf Nannmark, Göteborg University, Sweden.
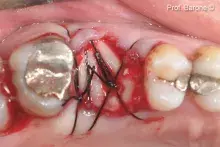
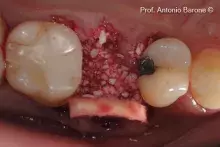
mp3® is ready-to-use, pre-hydrated and can be easily grafted into an antrostomy or post-extractive sockets.

Why choose mp3®?
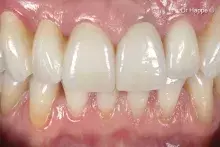
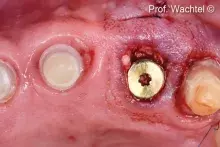
Although autogenous bone (AB) is still the gold standard for bone regeneration, it takes time to harvest it. Biomaterials are used as alternatives to AB, with the advantage of unlimited availability and reduced invasiveness. mp3® is a ready-to-use Dual-Phase biomaterial that can be directly syringed into sinus cavities, showing comparable results to autogenous bone chips¹⁻². Thanks to its similarity to natural human bone¹, mp3® has been easily syringed and successfully used in combination with Evolution membranes for alveolar ridge preservation, maintaining appropriate alveolar ridge volume and allowing a correct second-stage implant placement⁵. These unique properties allow a good graft volume preservation⁵, a healthy new bony tissue formation and ultimately, a successful implant rehabilitation over time⁶⁻⁸.
How to handle mp3®
mp3® must be used in a sterile environment. After adapting the material to the defect shape, it is necessary to remove non-stable residues before proceeding to the soft tissue suture.
Description:
- Collagenic bone mix granules pre-hydrated with collagen gel
- Heterologous bone mix
| Codes | Size | Type | Granulometry | Mean observed re-entry time |
|---|---|---|---|---|
| A3095FS | 0.5 cc | 1 Syringe | 600-1000 μm | About 5 months |
| A3005FS | 1 cc | 1 Syringe | 600-1000 μm | About 5 months |
| A3010FS | 2 cc | 1 Wide-tip syringe | 600-1000 μm | About 5 months |
| A3210FS | 2 cc | 1 Wide-tip syringe | 1000-2000 μm | About 5 months |
| Codes | Size | Type | Granulometry | Mean observed re-entry time |
|---|---|---|---|---|
| A3095FE | 0.5 cc | 1 Syringe | 600-1000 μm | About 5 months |
| A3005FE | 1 cc | 1 Syringe | 600-1000 μm | About 5 months |
| A3010FE | 2 cc | 1 Wide-tip syringe | 600-1000 μm | About 5 months |
| A3210FE | 2 cc | 1 Wide-tip syringe | 1000-2000 μm | About 5 months |
Clinical cases
Videos
Next Courses
Selected bibliography
Attention please! The OsteoBiol® website contains information on Medical Devices, which may be dangerous for the patient health and safety if not used exclusively by medical professionals.