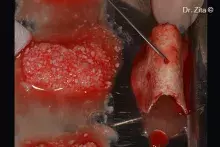
Putty
Engineered for peri-implant defects
The product
Putty is a pre-hydrated injectable bone paste containing collagenic bone granules with a granulometry of up to 300 μm.
The small diameter of the Dual-Phase granules facilitates the application of Putty into peri-implant lesions with intact bony walls¹. Putty should be used in defects able to firmly contain it, in order to stabilize the graft and allow its gradual resorption and new bone deposition.
Photomicrograph illustrating the healing after 5 weeks from Putty grafting in rabbits. Newly formed bone (#) can be detected around Dual-Phase bone granules (*) and close to the implant surface.
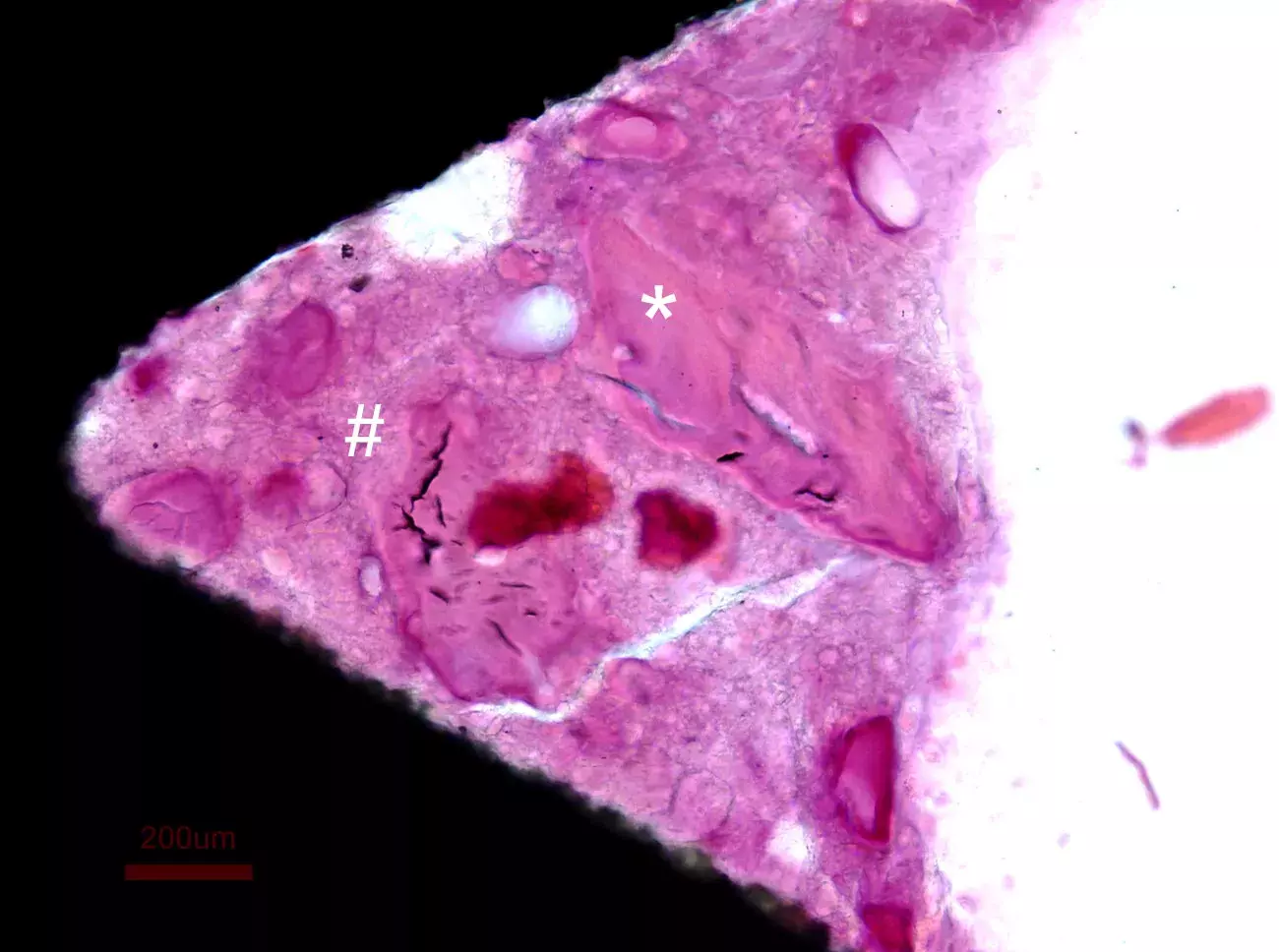
Courtesy of Prof. Ulf Nannmark, Göteborg University, Sweden.
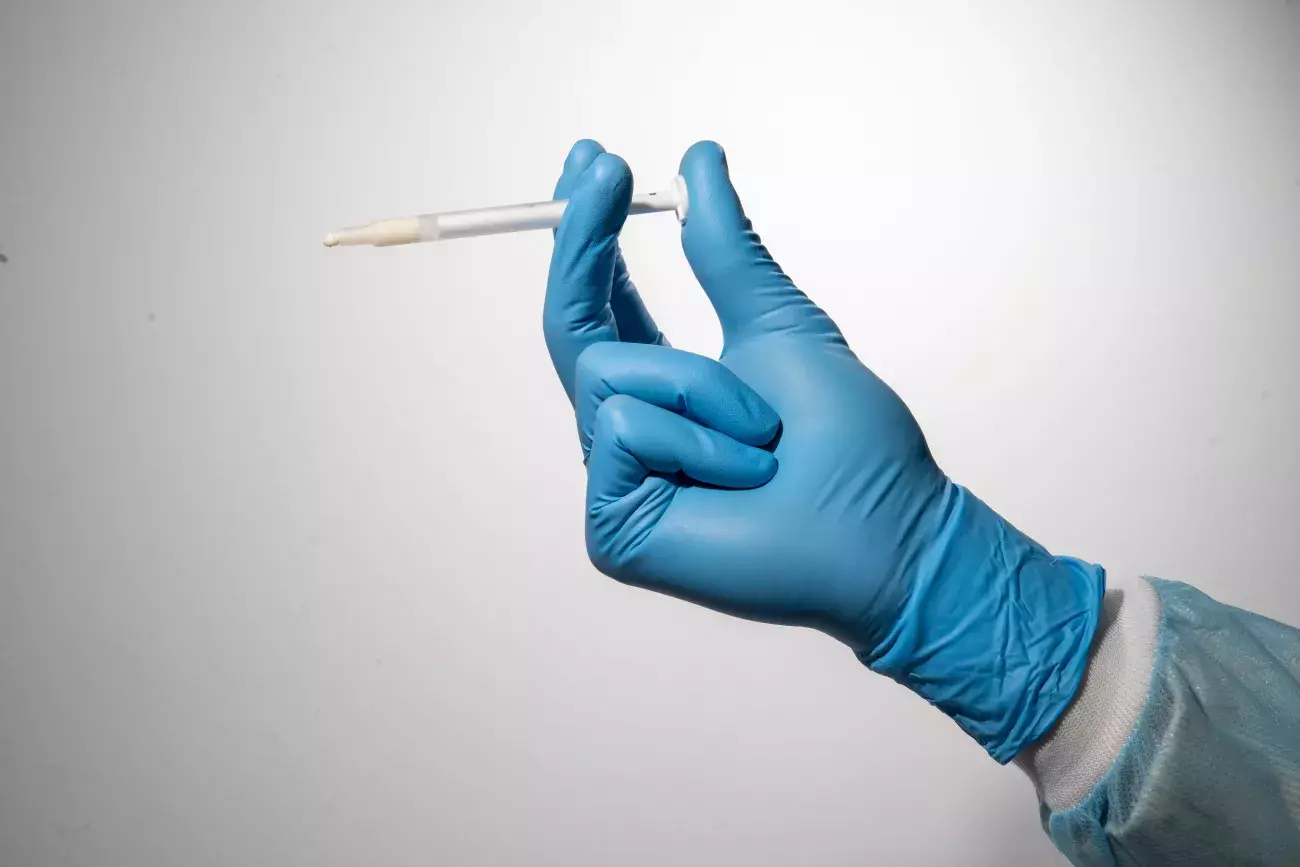
Putty is ready-to-use, pre-hydrated and can be easily inserted into narrow and self-containing defects.
Putty is a collagenic bone paste made of small-diameter heterologous bone granules and heterologous collagen gel.
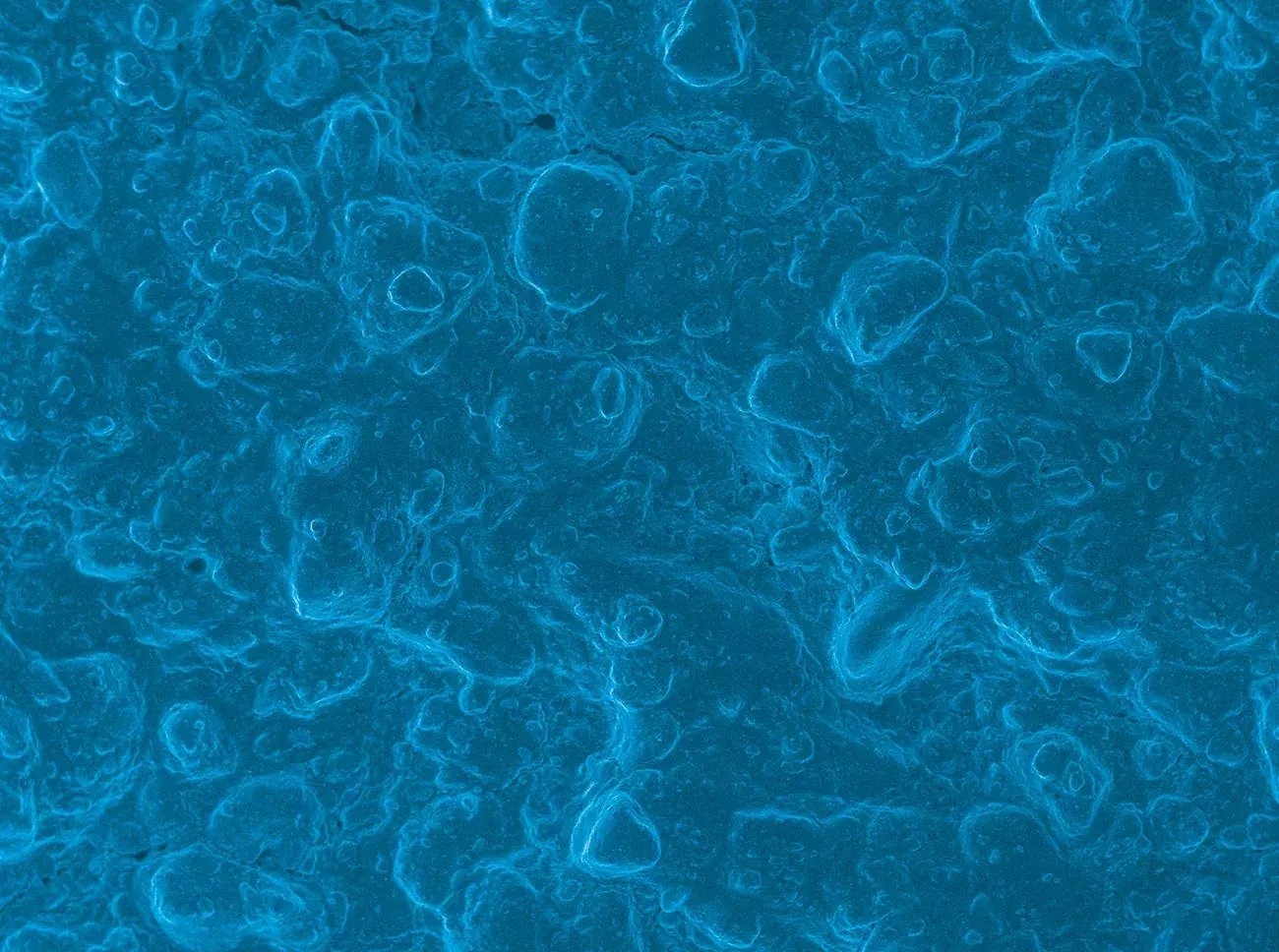
Source: Politecnico di Torino, Torino, Italy.

Why choose Putty?
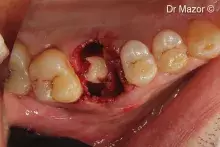
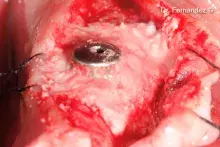
Peri-implant and small self-containing bone defects can be effectively grafted with a viscous biomaterial with small-diameter granules.
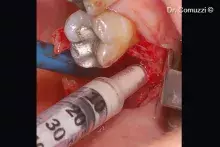
The “soft” consistency, the small-diameter bone granules of Putty and the practical ready-to-use syringe packaging make it a valid choice for small self-containing peri-implant lesions¹⁻² and small defects with a self-contained cavity. Thanks to its collagen gel content, Putty gently detaches the sinus membrane, thus being a valid option for transcrestal sinus floor elevation³ with immediate implant placement.
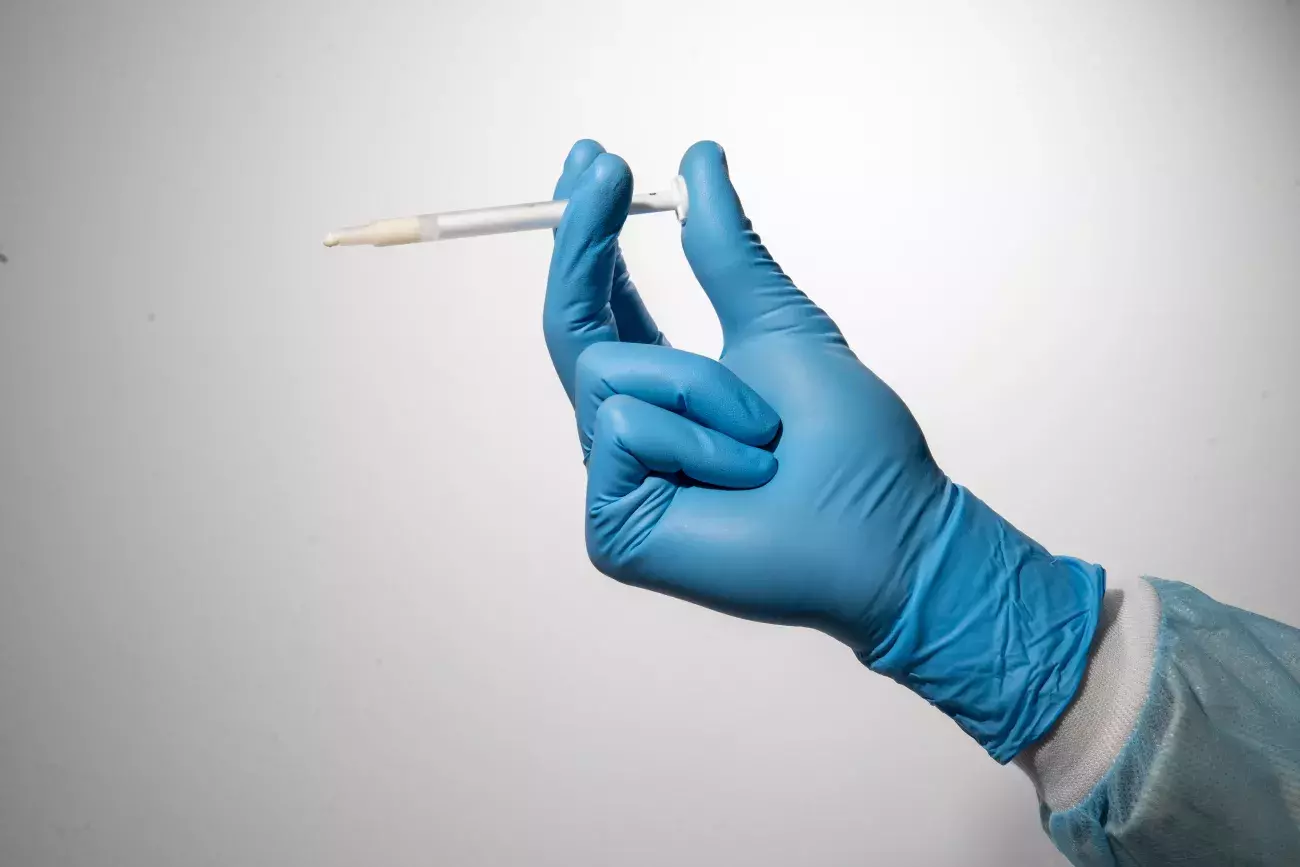
How to handle Putty
Putty should be injected into the defect and adapted to its morphology without extensive compression.
Any non-stable residue must be removed before soft tissue suture.
References
- Barone A et al. European Journal of Implant Prosthodontics, 2006, 2:99-106
- Cassetta M et al. Int J Periodontics Restorative Dent, 2012 Oct;32(5):581-9
- Saglanmak A et al. J Clin Med, 2024 Apr 11;13(8):2225
Description:
- Collagenic bone paste
- Heterologous cortico-cancellous bone mix
| Codes | Size | Type | Granulometry | Mean observed re-entry time |
|---|---|---|---|---|
| HPT52S | 0,25 cc | 1 Syringe | Up to 300 μm | About 4 months |
| HPT09S | 0,5 cc | 1 Syringe | Up to 300 μm | About 4 months |
| HPT61S | 1 cc | 1 Syringe | Up to 300 μm | About 4 months |
| Codes | Size | Type | Granulometry | Mean observed re-entry time |
|---|---|---|---|---|
| HPT52E | 0,25 cc | 1 Syringe | Up to 300 μm | About 4 months |
| HPT09E | 0,5 cc | 1 Syringe | Up to 300 μm | About 4 months |
| HPT61E | 1 cc | 1 Syringe | Up to 300 μm | About 4 months |
Clinical cases
Next Courses
Selected bibliography
Attention please! The OsteoBiol® website contains information on Medical Devices, which may be dangerous for the patient health and safety if not used exclusively by medical professionals.