Gen-Os®
The advantages of a Dual-Phase biomaterial
The product
Gen-Os® has a similar structure to human bone (matrix and porous form)¹ and presents osteoconductive properties²⁻³.
Thanks to its collagen content, Gen-Os® has chemotactic properties and facilitates blood clotting and the subsequent invasion of repairing and regenerative cells². In addition, it is gradually resorbable and provides appropriate support in bone neoformation⁴ and neo-angiogenesis⁵. Gen-Os® is a hydrophilic Dual-Phase xenograft, and it has been also used in association with growth factors⁶.
Photomicrograph illustrating the healing after 4 weeks from Gen-Os® grafting in rabbits. Newly formed bone (#) can be detected around and in between Dual-Phase bone granules (*).
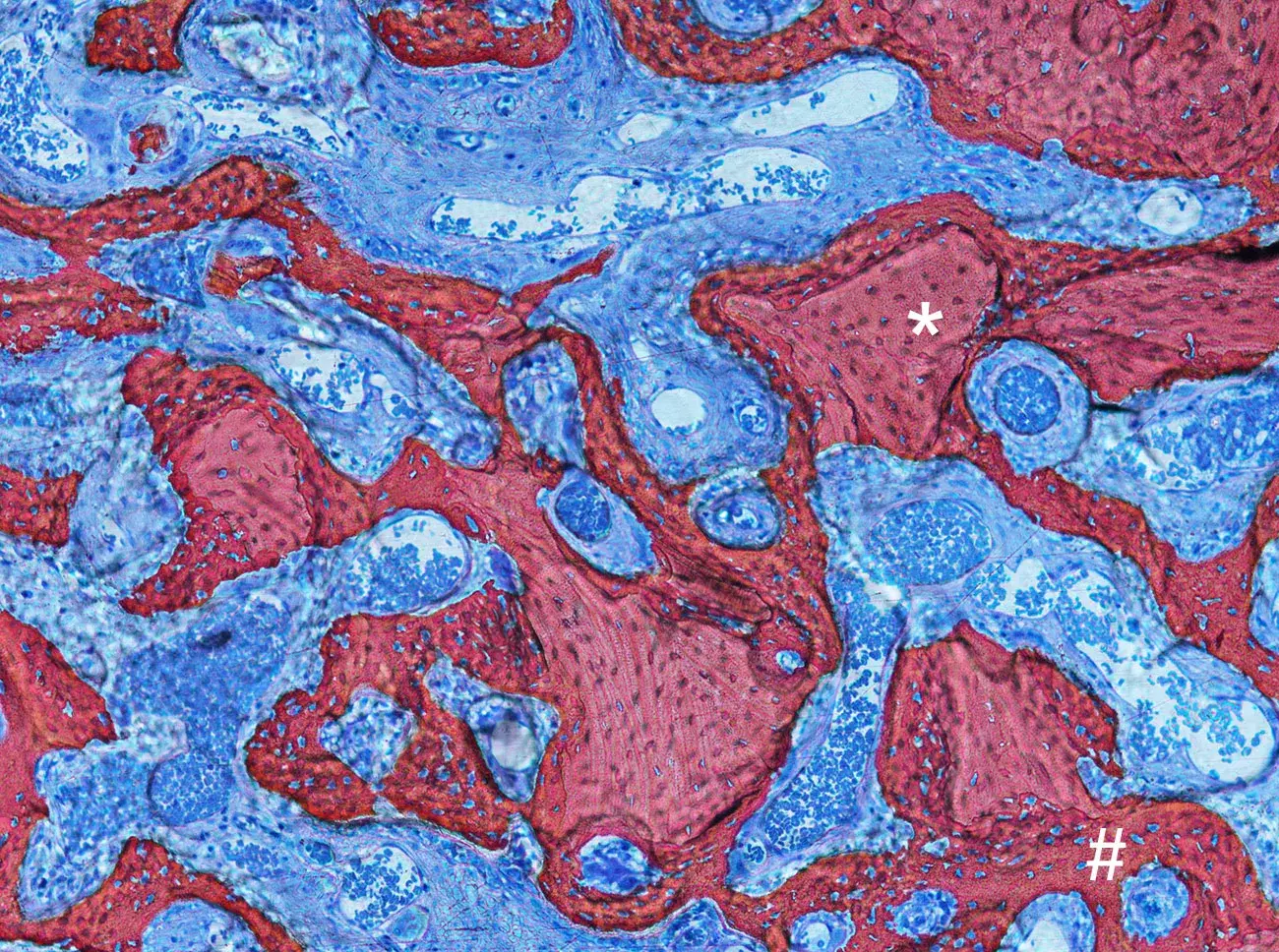
Courtesy of Prof. Daniele Botticelli, Scientific Director of ARDEC Academy, Italy
Gen-Os® is a dry cortico-cancellous collagenic bone mix.
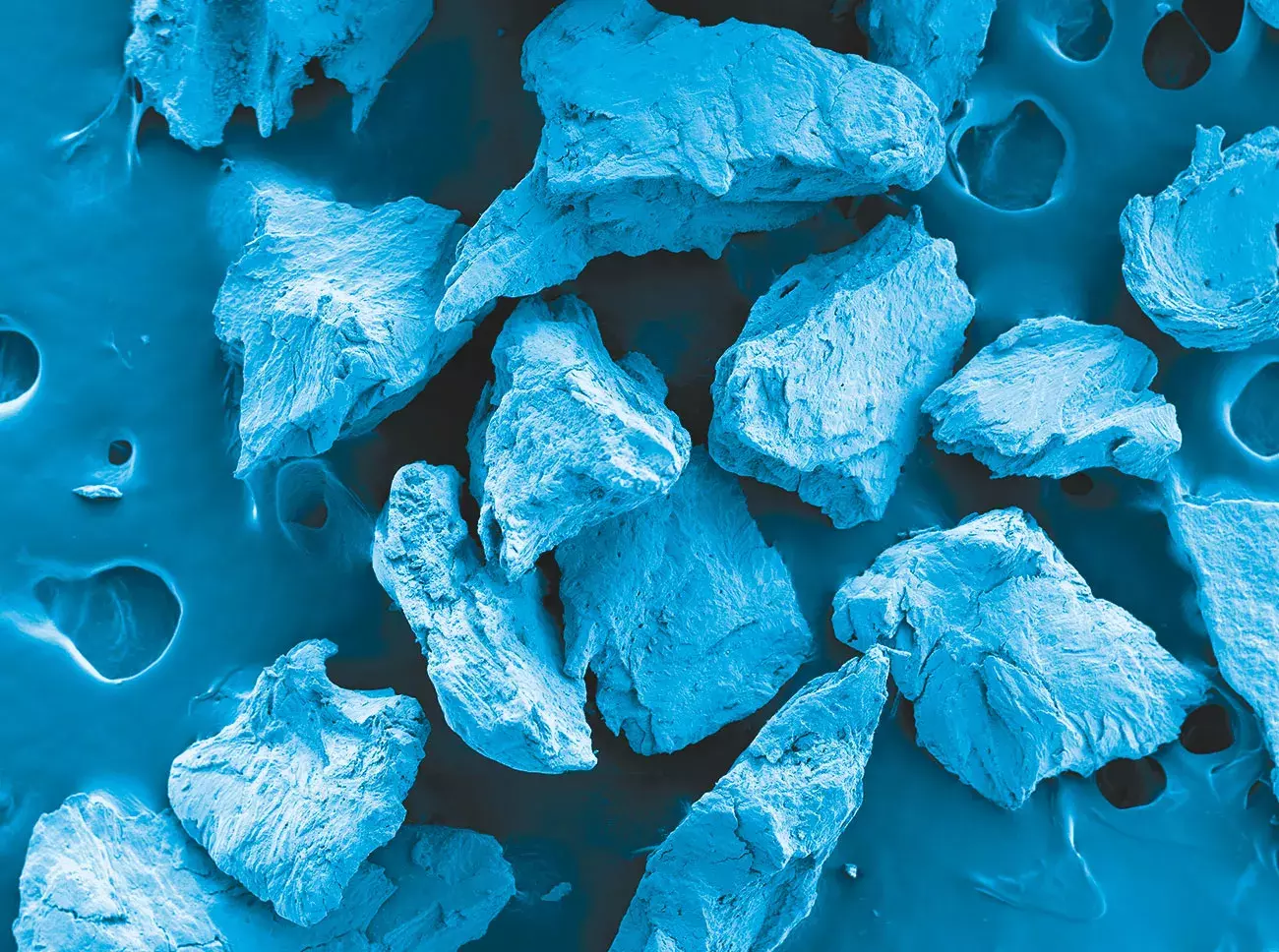
Source: Politecnico di Torino, Torino, Italy.
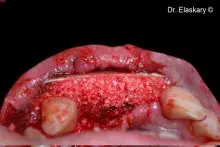
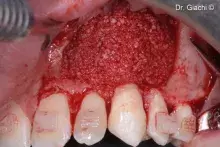
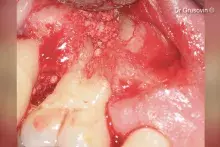
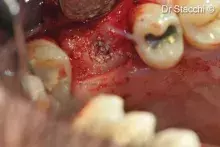
Gen-Os® can be mixed with patient's blood and can be used to effectively treat deep and narrow periodontal defects.

Why choose Gen-Os®?
Collagen is a precious protein for bone regeneration. Gen-Os® is the first Dual-Phase xenogenic bone substitute developed by Tecnoss® physically and chemically similar to autogenous bone¹. Gen-Os® can be used alone or mixed with AB chips whenever necessary⁷⁻⁹.
In addition, Gen-Os® has been successfully used for the treatment of deep and narrow periodontal defects⁴,¹⁰⁻¹³.
Finally, more than 25 years of clinical experience and about 100 scientific studies showed that Gen-Os® is a valid universal bone filler that allows predictable results in various surgical procedures³⁻¹⁴.

How to handle Gen-Os®
Gen-Os® must always be hydrated and thoroughly mixed with either a few drops of sterile physiological solution or the patient's blood to activate its collagen matrix embedded within the xenogenic bone granules.
References
- Figueiredo M et al. J Biomed Mater Res B Appl Biomater, 2010 Feb;92(2):409-419
- Nannmark U et al. Clin Implant Dent Relat Res, 2008 Dec;10(4):264-70
- Cassetta M et al. Clin Oral Implants Res, 2015 Oct;26(10):1180-4
- Cardaropoli D et al. Int J Periodontics Restorative Dent, 2009 Feb;29(1):59-67
- Rombouts C et al. Dent Mater J, 2016 Dec 1;35(6):900-907
- Mijiritsky E et al. Materials (Basel), 2017 Sep 8;10(9):1054
- Pellegrino G et al. Int J Oral Implantol (Berl), 2024 May 27;17(2):175-185
- Felice P et al. Int J Oral Implantol (Berl), 2024 Sep 16;17(3):285-296
- Elaskary A et al. BMC Oral Health, 2024 Oct 7;24(1):1190
- Esposito M et al. Eur J Oral Implantol, 2015 Autumn;8(3):233-44
- Aslan S et al. J Clin Periodontol, 2017 Sep;44(9):926-932
- Aslan S et al. Int J Periodontics Restorative Dent, 2017 Mar/Apr;37(2):227-233
- Kobe T et al. Clin Exp Dent Res, 2024 Feb;10(1):e853
- Romasco T et al. J Funct Biomater, 2022 Aug 18;13(3):121
Description:
- Heterologous dry cortico-cancellous collagenic bone mix
- Slightly radiopaque granules
| Codes | Size | Type | Granulometry | Mean observed re-entry time |
|---|---|---|---|---|
| M1052FS | 0.25 g | 1 Vial | 250-1000 μm | About 5 months |
| M1005FS | 0.50 g | 1 Vial | 250-1000 μm | About 5 months |
| M1010FS | 1.00 g | 1 Vial | 250-1000 μm | About 5 months |
| M1020FS | 2.00 g | 1 Vial | 250-1000 μm | About 5 months |
| M0210FS | 1.00 g | 1 Vial | 1000-2000 μm | About 5 months |
| M0220FS | 2.00 g | 1 Vial | 1000-2000 μm | About 5 months |
| Codes | Size | Type | Granulometry | Mean observed re-entry time |
|---|---|---|---|---|
| M1052FE | 0.25 g | 1 Vial | 250-1000 μm | About 5 months |
| M1005FE | 0.50 g | 1 Vial | 250-1000 μm | About 5 months |
| M1010FE | 1.00 g | 1 Vial | 250-1000 μm | About 5 months |
| M1020FE | 2.00 g | 1 Vial | 250-1000 μm | About 5 months |
Clinical cases
Next Courses
Selected bibliography
Attention please! The OsteoBiol® website contains information on Medical Devices, which may be dangerous for the patient health and safety if not used exclusively by medical professionals.