GTO®
GTO®
The sticky xenograft
The product
GTO® is sticky, gradually resorbable and osteoconductive¹.
Thanks to its high collagen content¹, GTO® allows an excellent rate of new bone formation²⁻⁴, delivering adequate graft volume preservation⁵, healthy new bone tissue and, ultimately, a successful implant rehabilitation²⁻⁶. GTO® is a mixture of heterologous collagenated cortico-cancellous granules and TSV Gel, the component that provides its sticky properties⁷.
Histology illustrating the healing after 4 months from GTO® grafting in human. GTO® Dual-Phase bone granules (*) are almost fully resorbed and replaced by an appropriate amount of newly formed bone (#).
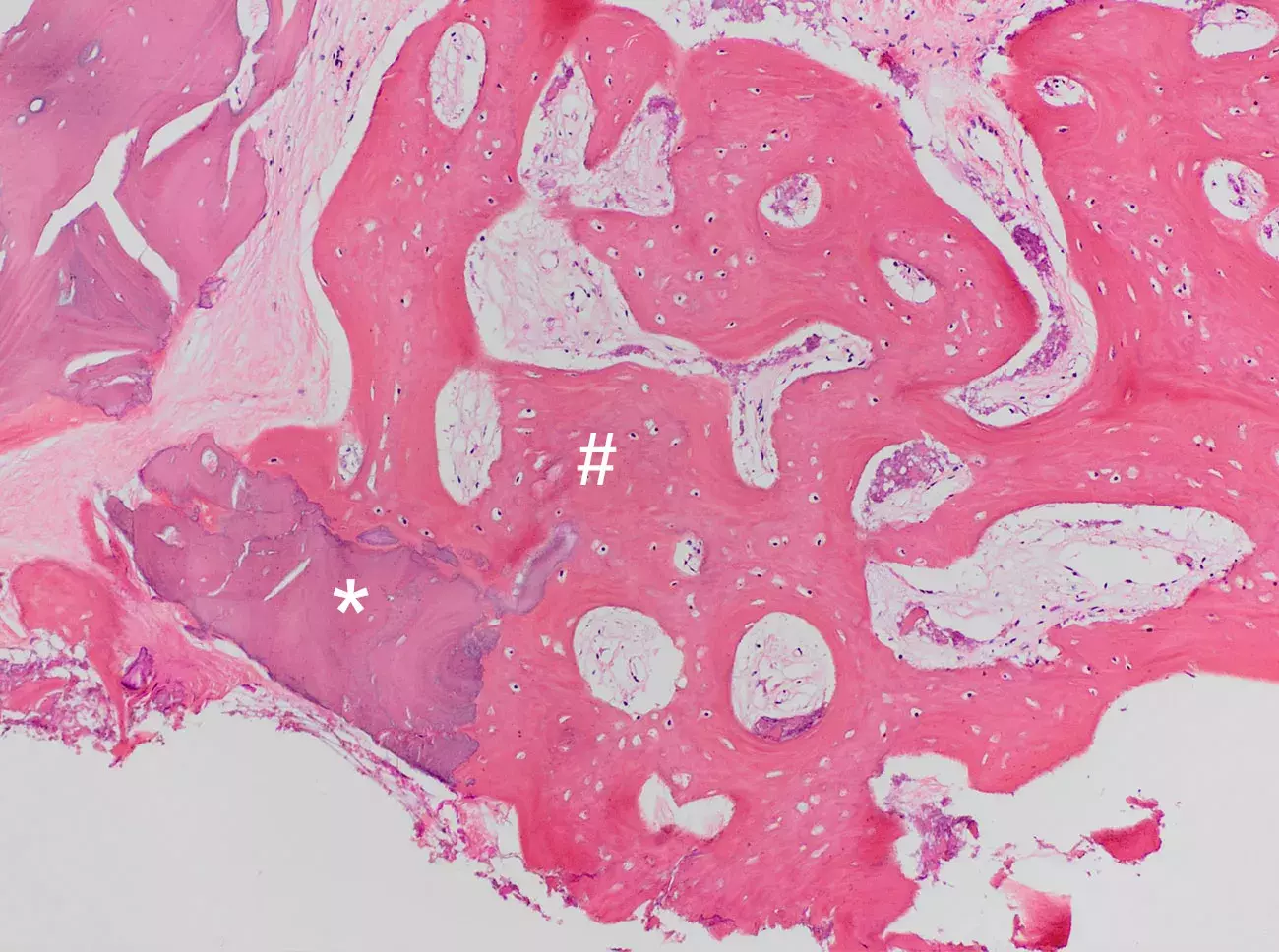
Courtesy of Dr. Paolo Savadori, Università di Modena e Reggio Emilia, Italy
GTO® is a collagenic sticky biomaterial made of a mix of heterologous bone granules and TSV gel.

Source: Politecnico di Torino, Torino, Italy
GTO® is sticky, ready-to-use, pre-hydrated, adaptable to the conformation of the defect and stable once grafted.

Why choosing GTO®?
Bone defect conformation varies among patients. GTO® is a Dual-Phase sticky xenograft characterised by high adaptability to the recipient site and high stability once grafted. GTO® allows adequate new bone formation²⁻⁴ and socket volume preservation when used for GBR⁵ in post-extractive sockets with delayed implant placement, even in the case of compromised post-extractive sockets. In combination with OsteoBiol® Lamina®, GTO® can also be the appropriate choice for the treatment of horizontal defects⁴ (i.e. knife-edge ridges). Thanks to its stickiness and ease of handling, GTO® can be used to regenerate bone around peri-implant dehiscences⁵.
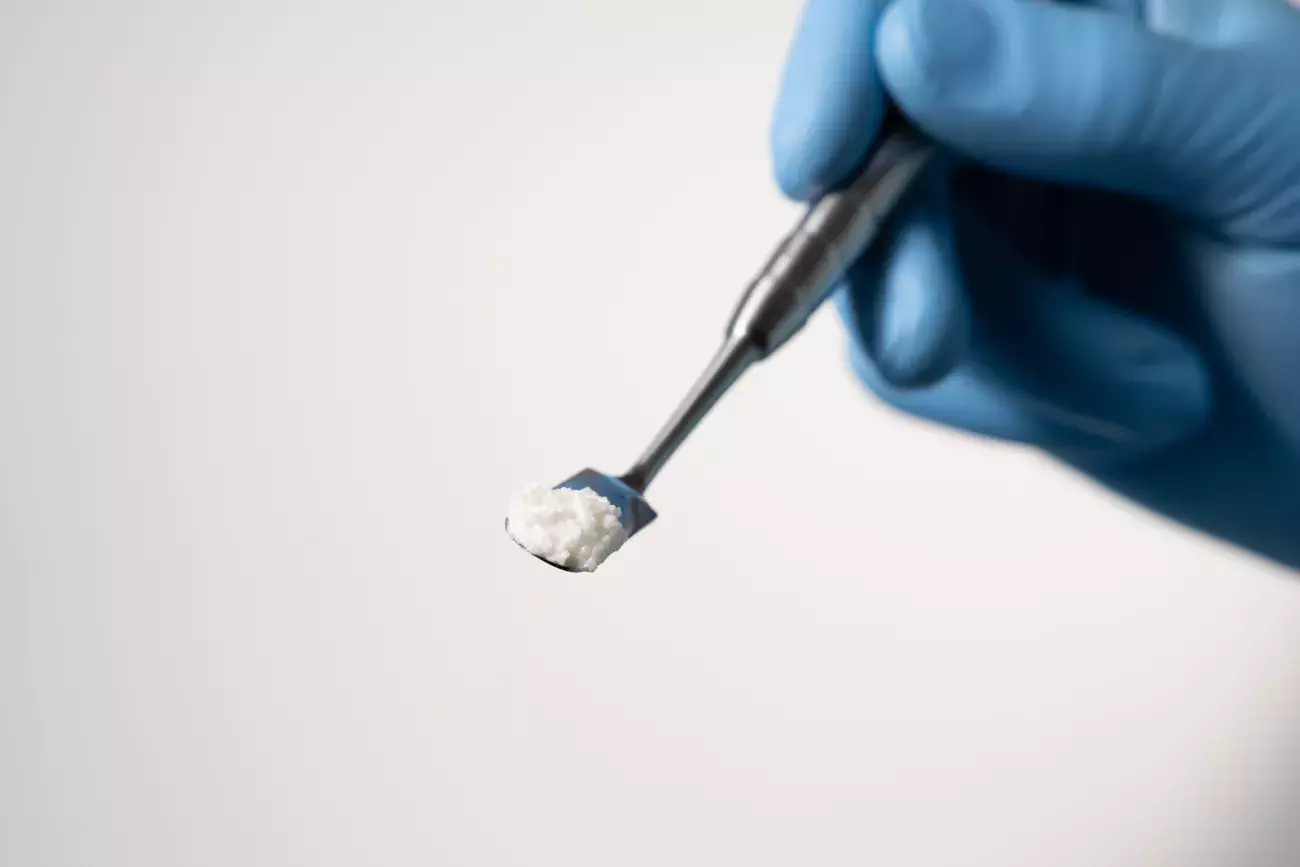
How to handle GTO®
GTO® is easily adaptable to any bone defect. It is ready-to-use, pre-hydrated and remains stable into the defect site. Thus, clinicians can skip the hydration step with saline or blood, saving time and decreasing the risk of accidental exposure to pathogens.
References
- Jeanneau C et al. Materials (Basel), 2024 Jan 27;17(3):625
- Canullo L et al. J Dent, 2024 Nov;150:105337
- Passarelli PC et al. Am J Dent, 2024 Sept;37(SIA):9A-12A
- Lopez MA et al. Am J Dent, 2024 Sept;37(SIA):41A-44A
- Menini M et al. Dent J (Basel), 2024 Jun 27;12(7):198
- Passarelli PC et al. Am J Dent, 2024 Sept;37(SIA):4A-8A
- Taniguchi Z et al. Int J Oral Maxillofac Implants, 2024 Oct 4;0(0):1-28
Description:
- Collagenic bone mix granules pre-hydrated with a thermosensitive copolymer
- Pre-hydrated granules and TSV Gel
- Heterologous bone mix
| Codes | Size | Type | Granulometry | Re-entry time |
|---|---|---|---|---|
| MU0005S | 0,5 cc | 1 Syringe | 600-1000 μm | 5 Months |
| MU0020S | 2,0 cc | 1 Wide tip syringe | 600-1000 μm | 5 Months |
| Codes | Size | Type | Granulometry | Re-entry tyme |
|---|---|---|---|---|
| MU0005E | 0,5 cc | 1 Syringe | 600-1000 μm | 5 Months |
| MU0020E | 2,0 cc | 1 Wide tip syringe | 600-1000 μm | 5 Months |
Clinical cases
Videos
Attention please! The OsteoBiol® website contains information on Medical Devices, which may be dangerous for the patient health and safety if not used exclusively by medical professionals.