A male patient (55 years old) requires horizontal and vertical augmentation
- GTO®
- Lamina®
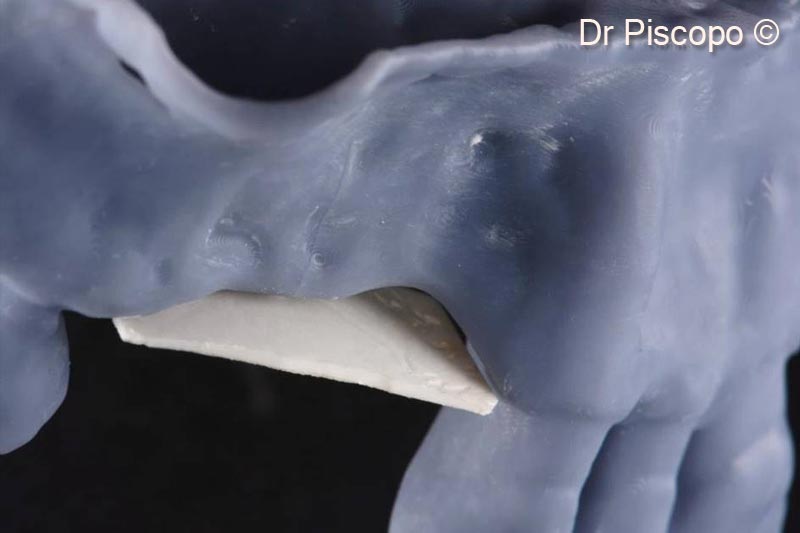
1. Oblique cranial view of the horizontal atrophic defect on a stereolithographic model
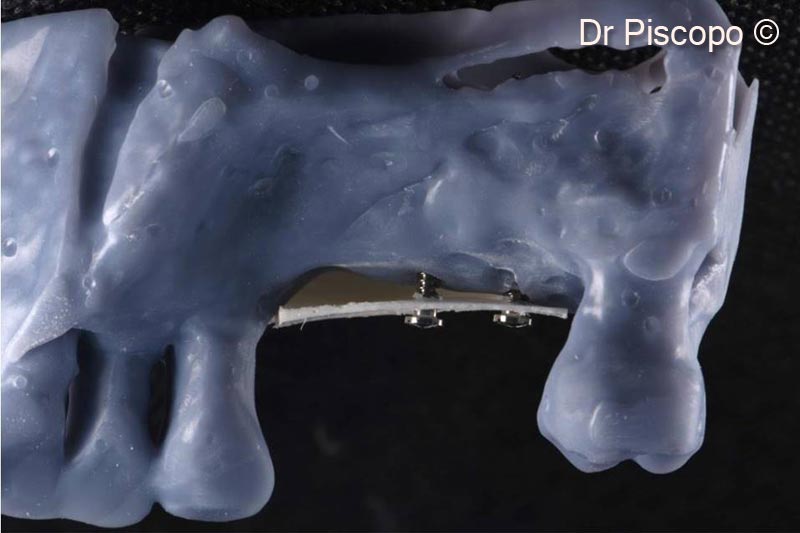
2. Lateral palatal view of the vertical atrophic defect on a stereolithographic model
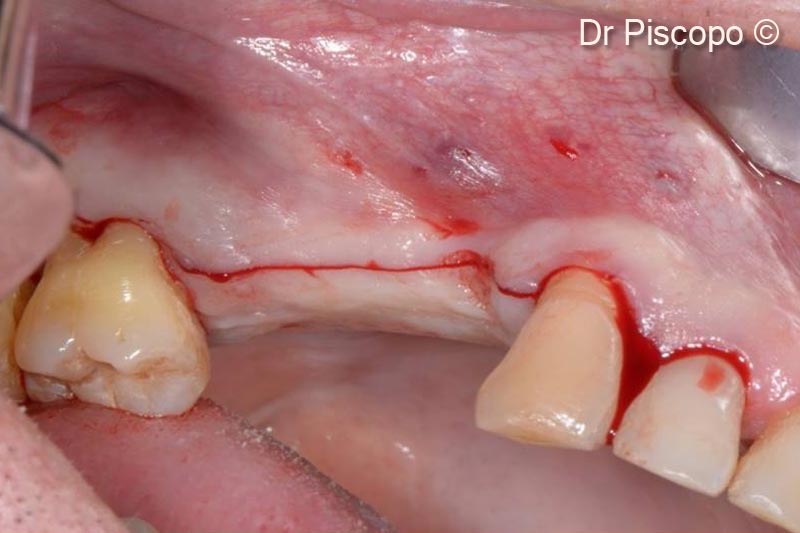
3. Vestibular supracrestal incision in order to ensure the amount of mucous tissue sufficient to cover the graft
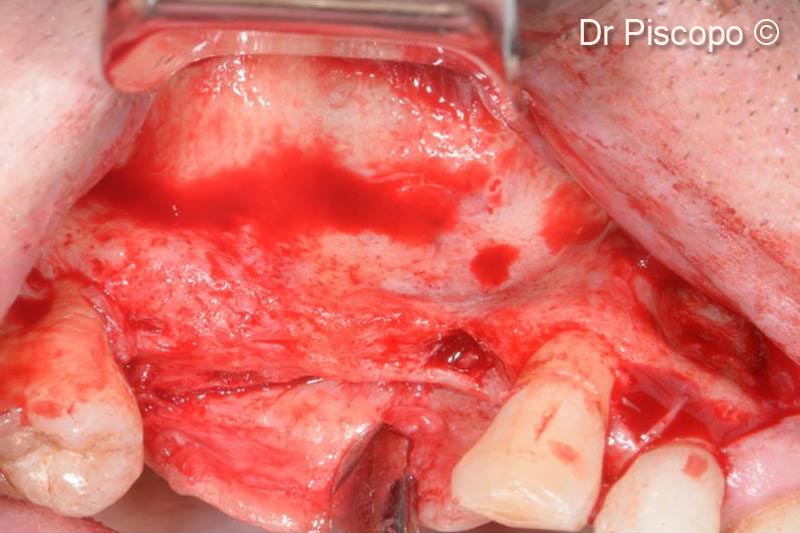
4. Preparation of the recipient site by means of a full-thickness trapezoidal flap
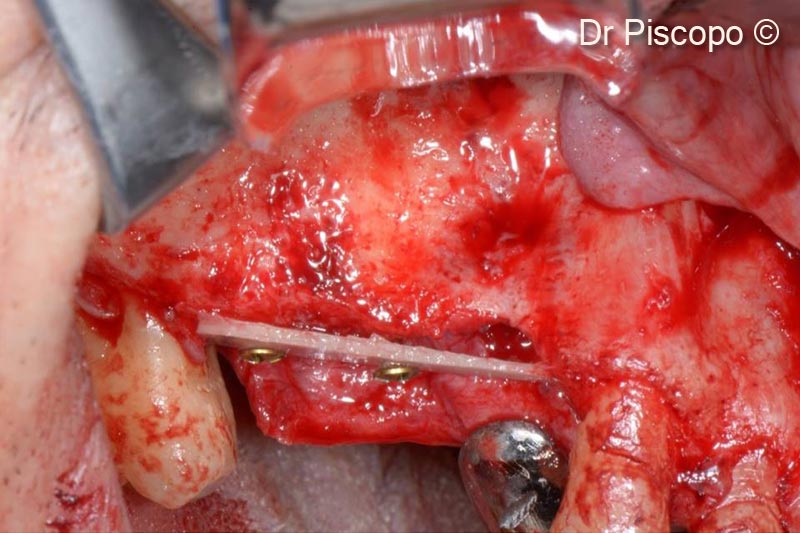
5. 1-mm thick Tecnoss® Lamina® positioned on the crestal side, to delimit the vertical atrophic defect, fixed with 1.3-mm osteosynthesis screws
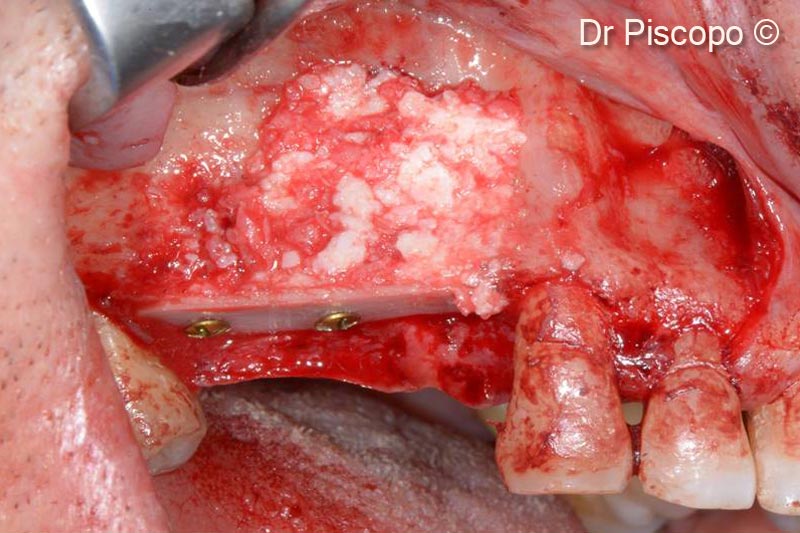
6. Atrophic defect filled with 50% OsteoBiol® GTO® and 50% autologous bone
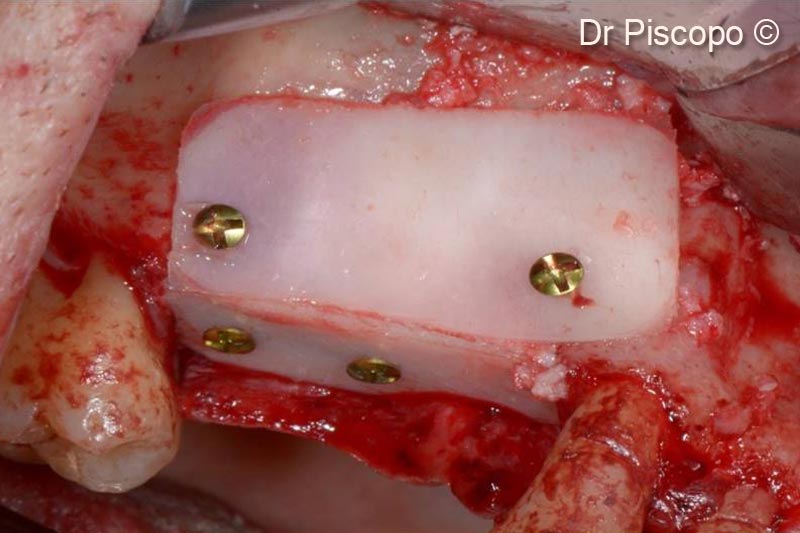
7. 1-mm thick Tecnoss® Lamina® positioned on the buccal side, to delimit the horizontal atrophic defect, fixed with 1.3-mm osteosynthesis screws
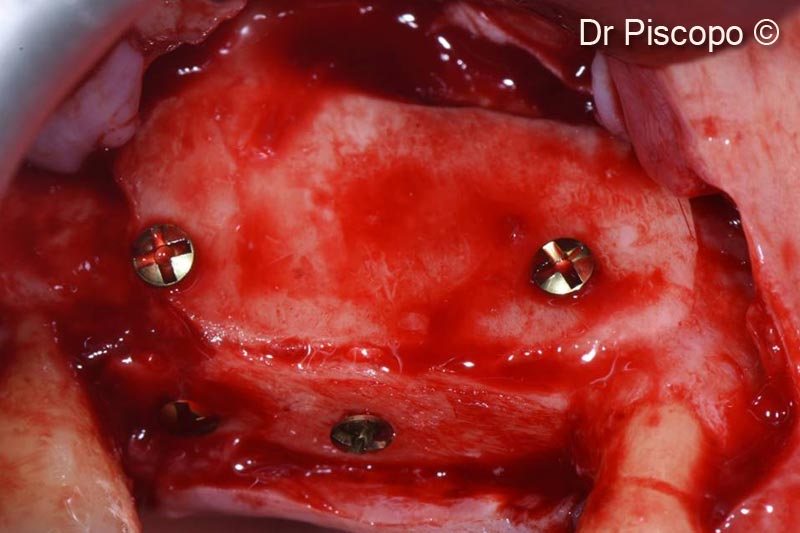
8. Second access performed 8 months after surgery
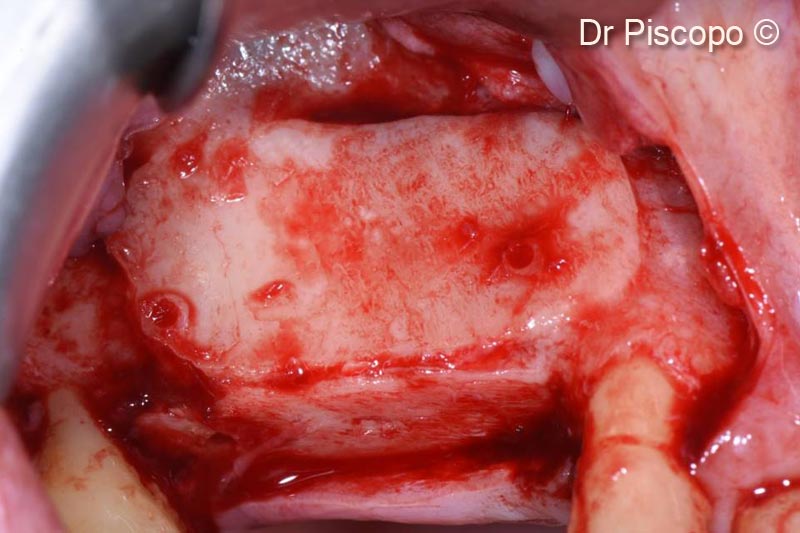
9. Removal of the osteosynthesis screws
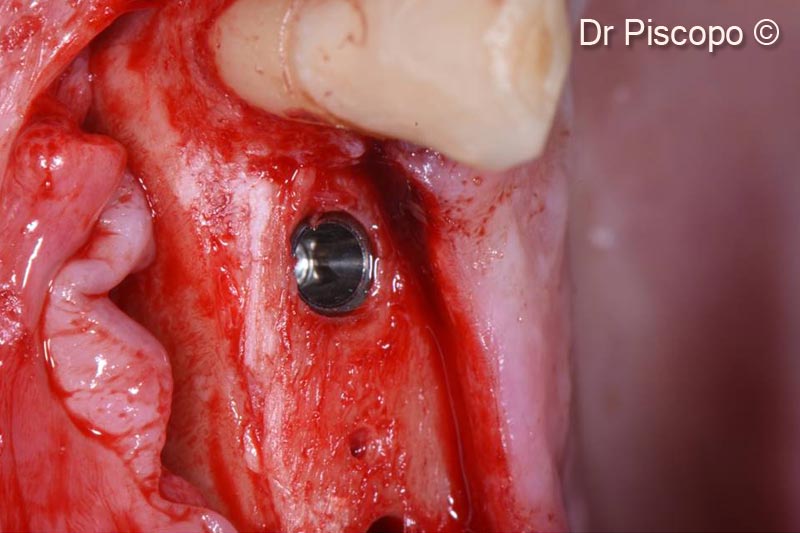
10. Insertion of 2 implant fixtures with 4x12 mm and 4x10 mm diameter
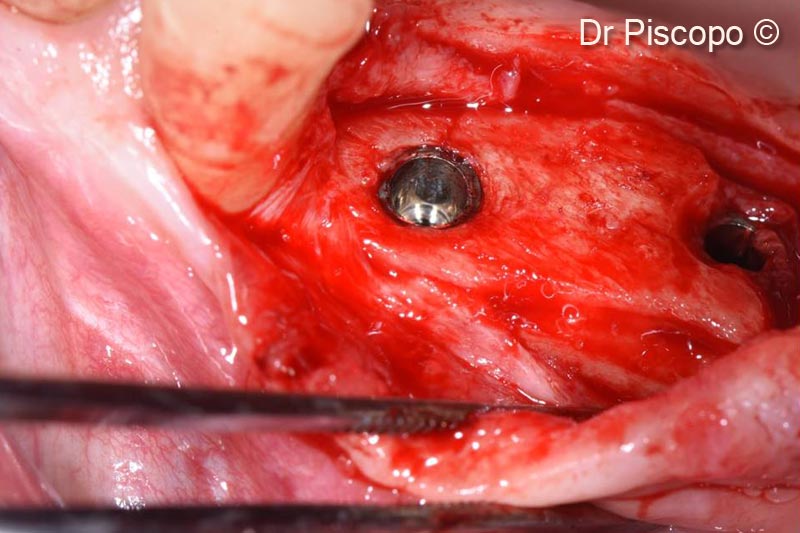
11. Surgical re-entry 4 months after implant positioning

